Psyttalia Walker, 1860
For additional information, especially on use in biological control, see the Psyttalia page on the website devoted to parasitoids of fruit-infesting Tephritidae: http://mx.speciesfile.org/projects/8/public/public_content/show/13190?content_template_id=88
Synonyms relevant to fruit-infesting tephritid parasitoids: Austroopius Szépligeti, 1900 (synonymized by Wharton 1987) and Acidoxanthopius Fischer, 1972 (synonymized by Wharton 1997).
Psyttalia was first recognized as an opiine by Muesebeck (1931), who synonymized it with Opius and renamed the type species walkeri because of secondary junior homonym in Opius. Prior to 1987, therefore, most species now in Psyttalia were placed in Opius. Psyttalia was treated as a subgenus of Opius by Fischer (1972), then elevated to generic rank by Wharton (1987). Wharton (1987) proposed Austroopius as a subgenus of Psyttalia, but later (Wharton 1997, 2009) abandoned subgenera due to lack of unambiguous features useful for defining each taxon as monophyletic. Wharton (1997) combined morphological and biological characters to suggest three informal species groups within Psyttalia, and also placed Acidoxanthopius as a synonym of Psyttalia.
Type locality of Psyttalia testacea: Sri Lanka; primary type in The Natural History Museum, London (Walker did not indicate how many specimens he had in the original description).
Western Indian Ocean
The two species occurring in Madagascar and the Mascarenes, Psyttalia insignipennis (Granger) and Psyttalia distinguenda (Granger) are readily separated from one another by the absence of an occipital carina in insignipennis. Additional information on hosts, distribution, and recognition, based on work done largely by CIRAD, was published by Wharton et al. (1999).
Palaearctic
A key for separating two of the Palaearctic species, Psyttalia ophthalmica (Tobias) and Psyttalia rhagoleticola (Sachtleben), from Psyttalia concolor can be found in Tobias (1977). Psyttalia ophthalmica is a darker species than the other two, with a slightly longer ovipositor and a coarsely punctate face. Psyttalia rhagoleticola has distinctly longer antennae than Psyttalia concolor (approximately 40 antennal segments in Psyttalia rhagoleticola and nearly always less than 35 antennal segments in Psyttalia concolor). Psyttalia ophthalmica is known only from the Maritime Region of far eastern Russia, around Vladivostok, where it has been reared from Rhagoletis in Lonicera and briars. Psyttalia rhagoleticola has a much more western distribution, occurring from western Europe to Kazakhstan, where it attacks various species of Rhagoletis, including Palaearctic cherry fruit fly, Rhagoletis cerasi. Psyttalia rhagoleticola also attacks Myoleja lucida (Fallén) larvae (Hoffmeister 1992). The distribution of Psyttalia rhagoleticola may overlap that of the other Palaearctic species, Psyttalia ponerophaga (Silvestri), known only from a single collection in Cherat, northern Pakistan, but the latter species was reared from olive fly in apparently wild olives. Psyttalia ponerophaga is one of the few parasitoids of fruit-infesting tephritids in this genus in which the fore wing m-cu enters the second submarginal cell. It is thus readily identified relative to the other 3 species discussed in this paragraph. Psyttalia concolor has been collected from olive fly, Bactrocera oleae, in Jordan (Mustafa and Al-Zaghal 1987) and Crete (Bigler et al. 1986).
Indo-Pacific
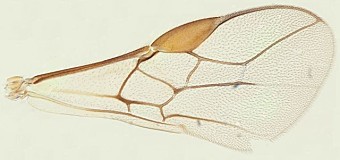
The 7 species from the Indo-Pacific region include 2 species, Psyttalia makii (Sonan) and Psyttalia walkeri (Muesebeck), with venation somewhat similar to that found in Psyttalia concolor (concolor, Fig. 1). The vein segment between m-cu and 2RS is usually distinctly thickened in part in Psyttalia makii but barely so or not at all in Psyttalia walkeri. The base of the second metasomal segment tends to be more polished in these two species than in Psyttalia concolor. Psyttalia makii was originally described from Taiwan but was subsequently recorded from the Philippines and Thailand. Psyttalia walkeri, originally collected in Sri Lanka, has also been recorded from Indonesia and Malaysia. Both species have been reared from various hosts in the Bactrocera dorsalis complex, as well as a few other species of Bactrocera.
The fletcheri species group. Three of these species, Psyttalia fijiensis, Psyttalia muesebecki, and Psyttalia novaguineensis have fore wing 2RS thickened medially (fijiensis, Fig. 2). The three species with a medially thickened 2RS have generally been separated from one another by coloration of the fore wing and sculpture of the propodeum (Fischer 1987), but both these features are somewhat variable. Psyttalia fijiensis tends to have the fore wing darkened medially while the fore wing of the other two species tends to be more uniformely hyaline. Psyttalia muesebecki apparently lacks a median carina on the propodeum but this is present in Psyttalia novaguineensis. Psyttalia fijiensis is more widely distributed than the other two, and consequently has a wider range of hosts (Wharton and Gilstrap 1983). Psyttalia muesebecki is known only from New Caledonia.
The two remaining species, Psyttalia fletcheri and Psyttalia incisi, are very similar to one another in the curvature of fore wing m-cu and distally enlarged subdiscal cell, but m-cu is more basally displaced in Psyttalia incisi, resulting in a different pattern of thickening ( incisi, Fig. 2 and fletcheri, Fig. 1). Both species were originally described from India and subsequently introduced to and established in Hawaii. Psyttalia fletcheri attacks the melon fly, Bactrocera cucurbitae and Psyttalia incisi attacks Oriental fruit fly, Bactrocera dorsalis. Additional host and distribution records can be found in Wharton and Gilstrap (1983).
Afrotropics
Two species, Psyttalia cosyrae (Wilkinson) and Psyttalia phaeostigma (Wilkinson) have distinctly longer ovipositors (cosyrae, Fig. 6) than all of the others. Psyttalia cosyrae has been reared from Ceratitis cosyra infesting mangoes and a few wild host plants in Kenya and Tanzania. Psyttalia phaeostigma has been reared from Dacus ciliatus infesting cucurbits in Kenya. Psyttalia lounsburyi has a distinctly darker color pattern than most other Afrotropical species of Psyttalia. Considerable information on speciation in the Afrotropics can be found in Rugman-Jones et al. (2009).
There are two morphologically distinct, informal groups within the genus as first noted by Fischer (1972). The first group corresponds largely with Fischer’s (1972, 1987) group A and includes the Psyttalia concolor and P. fletcheri species groups of Wharton (1997), as well as species formerly included in Austroopius and Opius ( Acidoxanthopius ). Available host records suggest that members of this first group are almost exclusively parasitoids of fruit-infesting Tephritidae. The second group largely corresponding to Fischer’s (1972, 1987) group B and includes the Psyttalia vittator (Brues) species group of Wharton (1997). The vittator species group, attacking tephritids in flower heads, is not further discussed here.
There are numerous species of fruit-infesting tephritid parasitoids in the genus Psyttalia, and further attempts to delineate species groups are warranted. I continue to recognize the Psyttalia fletcheri species group and include the following in this group: P. amboiensis (Fullaway), P. fijiensis (Fullaway), P. fletcheri, P. incisi, P. lemiensis (Szépligeti), P. muesebecki (Fischer), and P. novaguineensis (Szépligeti). Most of these species have 2RS thickened medially, the sole character traditionally used to define Austroopius (Szépligeti 1900; Fischer 1972, 1987; Wharton and Gilstrap 1983). However, I define the fletcheri species group more broadly to include species such as fletcheri and incisi, while specifically excluding P. insignipennis (Granger). Members of the fletcheri species group are distinguished from all other Psyttalia on the basis of the wide angle formed between fore wing m-cu and 1CUb, with m-cu often broadly bowed towards 2RS. The first subdiscal cell is nearly always distinctly broadened distally in association with this diagnostic feature. Known members of the fletcheri species group are Indo-Pacific in distribution, and I have seen several undescribed species distributed from northeastern Australia through Malaysia which differ from described species primarily on the basis of differences in ovipositor length and fore wing venation. Intraspecific variation in thickness of 2RS is known both for species within the fletcheri species group (Wharton and Gilstrap 1983) and for species in other species groups (Wharton et al. 1999). Hosts are unknown for P. amboiensis and P. lemiensis, but all other members of the fletcheri species group are parasitoids of various species of Bactrocera Macquart (Silvestri 1916; Clausen et al. 1965; Carmichael et al. 2005). These hosts have previously been placed in Dacus Fabricius, but I know of no confirmed records from Dacus as the latter genus is currently (2009) defined. Similarly, records of P. incisi from Carpomya vesuviana Costa (e. g. Wharton and Gilstrap 1983) need confirmation since these records apparently come from fruit samples with mixed infestations of C. vesuviana and Bactrocera spp., whereas large samples yielding only C. vesuviana failed to produce P. incisi (e. g. Clausen et al. 1965). Psyttalia novaguineensis has been recorded from several species of Bactrocera (Fischer 1987; Carmichael et al. 2005), and the sole record from Euphranta Loew (Fischer 1987) should also be verified.
Psyttalia acidoxanthicida (Fullaway) is a distinctive species for which Fischer (1972b) created the subgenus Acidoxanthopius. Vein 1M of the hind wing is somewhat thickened and distinctly bowed, yet the venation of the fore and hind wing is otherwise typical of Psyttalia. Distal expansion of the first subdiscal cell and more equal length of T2 and T3 suggest a placement closer to the fletcheri species group than to the concolor species group. Though T2 is shorter than T3 in Psyttalia, the relative length varies substantially with T2 very short in many of the African species and distinctly longer in many of the species in the fletcheri group. Psyttalia acidoxanthicida is further characterized by a reduced occipital carina and reduced sculpture on the propodeum and petiole. Psyttalia acidoxanthicida was reared from Acidoxantha Hendel infesting buds of Hibiscus L. The host is thus atypical relative to known hosts of the fletcheri and concolor species groups, which, with only one exception noted below, attack fruits rather than buds.
Most of the remaining species of Psyttalia differ from one another by relatively smooth transitions in color, ovipositor length, sculpture, and venation. I am unable to divide them satisfactorily into distinct species groups despite several attempts to delineate a smaller subset as the concolor species group (Wharton and Gilstrap 1983; Wharton 1997; Kimani-Njogu et al. 2001; Rugman-Jones et al. 2009). Species such as P. concolor, P. humilis, P. cosyrae (Wilkinson), P. perproxima (Silvestri), and P. phaeostigma (Wilkinson) are capable of hybridizing (producing viable female offspring) when confined in small containers (Kimani-Njogu et al. 2001; Billah et al. 2008a) despite originating from genetically distinct populations and in some cases being morphologically distinct as well (Kimani-Njogu et al. 2001; Billah et al. 2008b; Rugman-Jones et al. 2009). Several cryptic species remain to be elucidated, and applying available names to various Afrotropical populations will continue to be a challenge (Rugman-Jones et al. 2009). My dissections of the reproductive tract reveal that these particular species also have a distinctive bulb associated with the venom apparatus that, in P. concolor at least, is filled with virus particles (Wharton 1997). In addition to these five species, a similar bulb is present in P. halidayi. The venom apparatus of P. lounsburyi (Silvestri) and P. masneri are different, however, and I was unable to find a similar feature in females of the two specimens of lounsburyi and the one of masneri that I dissected. The bulb is also absent in four species of the fletcheri species group, P. fijiensis, P. fletcheri, P. incisi, and P. novaguineensis (Wharton 1997; Quicke et al. 1997), as well as in several other species examined by Quicke et al. (1997).
Until more work can be done on the venom apparatus, and perhaps hybridization experiments, I reluctantly include a large number of species in the concolor species group at the present time. Wharton (1997) and Rugman-Jones et al. (2009) already noted the similarity of P. dacicida (Silvestri), P. dexter (Silvestri), and P. ponerophaga (Silvestri) to the species with a bulb on the venom apparatus, noted above (P. concolor, P. cosyrae, P. halidayi, P. humilis, P. perproxima, and P. phaeostigma). Of the remaining species with host records, or at least reared from fruits, P. efoveolata (Szépligeti), P. makii (Sonan), P. ophthalmica (Tobias), P. rhagoleticola, and P. walkeri (Muesebeck) are also morphologically similar, whereas P. distinguenda (Granger) and P. insignipennis, both originally described from Madagascar, and P. inquirenda (Silvestri) and P. inconsueta (Silvestri), from Cameroon and Nigeria respectively, have (RS+M)b less distinct and often absent. Psyttalia somereni (Fischer) and P. lounsburyi are distinctly darker species, and Fischer (1987) separates P. somereni from most other species on the basis of the absence of a median keel on the extensively rugose propodeum. I have not seen P. brevitemporalis, another species with a host record, but the original description is consistent with membership in the concolor species group. Based on examination of type specimens, I include the following in the concolor species group and predict that they also attack fruit-infesting tephritids: P. bisulcata (Szépligeti), P. haemaelaeineni Fischer, P. hemicauda (Fischer), P. palpalpis (Szépligeti), P. pusilla (Szépligeti), P. tshuapana (Fischer), and P. yangambiana (Fischer). Biological information is also lacking for the following species, but based on the descriptions by Tobias (1998) and redescriptions by Fischer (1987), I include them in the concolor species group: P. alleni, P. cyclogastroides, P. darasunica, P. romani, P. sakhalinica, and P. vacua.
There are two species that have fore wing venation typical of the concolor species group, but sculptural features of the vittator species group. Psyttalia masneri is more heavily sculptured than nearly all other species reared from fruit-infesting tephritids and shares sculptural and other features with P. paralleni (Fischer). I place them together in the paralleni species group based specifically on the distinctly sculptured lower gena and narrower clypeus relative to members of the concolor species group.
Extensive information is available on the biology of Psyttalia concolor (Szépligeti), P. humilis (Silvestri), and P. fletcheri (Silvestri) in association with their use in biological control. More limited information is available on P. incisi (Silvestri) in this regard. Psyttalia concolor was introduced to Italy in an effort to control olive fly shortly after its discovery in Tunisia. Its early use in Italy has been well documented (Silvestri, 1922, 1938; Delucchi, 1957), as has its subsequent use in augmentation programs following development of mass rearing techniques using medfly as hosts. As a result of these efforts, there is now a considerable amount of information on the developmental biology of Psyttalia concolor, as well as other facets of its biology related to its utility for biological control of fruit pests (Féron, 1954; Ragusa, 1957; Biliotti and Delanoue, 1959; Delanoue, 1960, 1961; Arambourg, 1962; Genduso, 1967; Liotta, 1969; Raspi and Loni, 1994; Loni, 1997; Canale, 1998; Raspi and Canale, 2000; Canale and Raspi, 2000). P. concolor and P. lounsburyi have been reared from Bactrocera oleae collected from Olea europaea cuspidata in Kenya (Copeland et al. 2004). In experimental studies, P. concolor successfully parasitized second instar larvae of Ceratitis capitata (Wiedemann), and first and second instar larvae of Bactrocera oleae (Rossi) (Canale 1998, Raspi and Canale 2000). P. concolor siculus was introduced in Bolivia in 1968 to control Ceratitis capitata (Bennett and Squire 1972).
Psyttalia humilis, originally collected in South Africa, was successfully established in Hawaii in 1913 against medfly and a detailed biology was published by Pemberton and Willard (1918).
During the exploration phase of the Oriental fruit fly program, several of the opiines from Kenya were variously identified as color varieties of Psyttalia concolor or as Psyttalia perproxima (Clausen et al. 1965). Material from the same localities had been identified as either P. humilis or P. perproxima during an earlier sampling program (Bianchi and Krauss, 1936). Difficulty in identification of these three species is still a problem, and uncertainty over whether or not they are distinct makes it difficult to correctly associate previously published host records. See Rugman-Jones et al. (2009) for a more detailed treatment of this problem.
Much of the biological information on Psyttalia fletcheri and Psyttalia incisi is summarized by Clausen (1978). More recent information can be found in Ramadan et al. (1991). Other species, originating from Kenya, are currently being cultured in France, Guatemala, California, and Hawaii; see species pages for Psyttalia humilis , Psyttalia concolor , Psyttalia lounsburyi , and Psyttalia ponerophaga .
There are numerous host records for members of the expanded concolor species group as defined here. A few species, notably P. efoveolata, P. inquirenda, P. somereni, and P. walkeri, have been reared only from fruits, with the host fly unknown (Silvestri 1913; Fischer 1972a, b, c). Three of the species, P. dacicida, P. lounsburyi, and P. ponerophaga, are parasitoids of olive fly, B. oleae (Silvestri 1912, 1913, 1916b; Copeland et al. 2004; Sime et al. 2007; Daane et al. 2008), and have thus far been recorded only from this host. Psyttalia concolor is also a parasitoid of B. oleae and was originally described from specimens reared from olives. It is capable of attacking a wide variety of other fruit-infesting tephritids both in its native range and in areas where it has been introduced. In addition to Bactrocera, known hosts include tephritid species in the genera Anastrepha Schiner, Ceratitis MacLeay, Capparimyia Bezzi, Carpomya Costa, and Dacus Fabricius (Wharton and Gilstrap 1983). Psyttalia makii has been recorded from both Bactrocera and Carpomya (Wharton and Gilstrap 1983) while P. dexter, P. perproxima, and P. phaeostigma have all been reared from various species of Dacus ( SIlvestri 1913; Steck et al. 1986; Kimani-Njogu et al. 2001). Psyttalia perproxima is primarily a parasitoid of various Ceratitis and Trirhithrum Bezzi species while P. phaeostigma, which is mainly known as a parasitoid of Dacus ciliatus Loew and other cucurbit pests, has additionally been recorded from Ceratitis and Carpophthoromyia. Psyttalia cosyrae, P. distinguenda, P. humilis, and P. insignipennis have all been reared from species of Ceratitis (Silvestri 1913; Wilkinson 1927; Wharton et al. 1999; Mohamed et al. 2003, though insignipennis may have a broader host range ( Wharton et al. 1999) and humilis may have been reared on other hosts at least briefly during attempts to redistribute it from Hawaii for biological control of other tephritid pests (Clausen 1978; Wharton 1989). For a recent species-level treatment with additional host records, see Rugman-Jones et al. (2009). The temperate species P. ophthalmica and P. rhagoleticola are both parasitoids of Rhagoletis ( FIscher 1972b; Tobias 1977) and P. brevitemporalis was described from specimens reared from a species of Myoleja ( Tobias (1998)). Finally, Silvestri (1913) recorded P. inconsueta from Carpophthoromyia tritea Walker. Though Fischer (1987) placed inconsueta in his group B, based on the wing venation as illustrated by Silvestri (1913), the species is otherwise more similar in sculpture and facial features to other members of the concolor species group, and at least one of the wings on the type series has fore wing m-cu interstitial rather than postfurcal.Although the hosts recorded above for the concolor species group are fruit-infesting tephritids, the only known host of P. dexter develops in fruits that are pod-like (Silvestri 1913). Similarly, I have seen specimens that are not easily distinguished from P. concolor, reared from Coelotrypes Bezzi infesting flowers of Convolvulaceae. Thus, a few caveats need to be attached to the generalizations about the types of hosts attacked by members of the concolor species group. Also, because of the evidence for host associated differentiation in this group, as exemplified by P. halidayi, published host records need to be carefully verified.
Psyttalia humilis, originally collected in South Africa, was successfully established in Hawaii in 1913 against medfly. The efficacy of Psyttalia humilis and three other introduced species was extensively documented over a 23 year period (Pemberton and Willard, 1918; Willard and Mason, 1937), and detailed biologies were published by Pemberton and Willard (1918). All four species were still established in 1933, the last year for which data are available from this program, with Psyttalia humilis and Diachasmimorpha tryoni (Cameron) exhibiting roughly equivalent levels of parasitism on medfly. The principle benefit of these introductions was the considerable reduction in infestation levels in coffee, which had previously been so badly infested that the coffee berries could not ripen (Willard and Mason, 1937). Infestations in other fruits was also reduced, though not as much in large, fleshy, preferred fruits such as mango. Nevertheless, reduced infestations made it possible to integrate other control measures more successfully, and eliminate medfly in some non-preferred fruits. Psyttalia humilis was last recorded from Hawaii in 1933 and subsequently disappeared for reasons unknown.