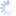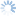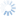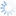Diachasmimorpha tryoni (Cameron, 1911)
Biosteres acidusae Fischer, 1967 is a synonym of tryoni (Wharton and Marsh 1978, Wharton and Gilstrap 1983).
Introduced to Hawaii, Florida, California, and several places in Latin America but in most places outside Hawaii, either failed to establish or establishment has not been confirmed. Guatemala is a relatively recent exception.
The first records of Diachasmimorpha tryoni were apparently by Gurney (1910), (1911) in Australia who noted that it developed on both the native host, Queensland fruit fly, as well as the introduced pest Mediterranean fruit fly. May and Kleinschmidt (1954) placed D. tryoni in perspective relative to other opiine parasitoids of Tephritidae in Queensland. Jessup and Walsh (1998) examined diapause in lab cultures that had been recently collected in New South Wales.
In interspecific competition studies with Fopius arisanus (Sonan), a tephritid egg-prepupal parasitoid, Wang and Messing (2003), found that D. tryoni rarely survived in hosts previously parasitized by F. arisanus. The authors suggest that competition from F. arisanus in regions of Hawaii where both parasitoids have been introduced may be driving D. tryoni to select nontarget host species. Earlier work (summarized in Willard and Mason 1937) showed effect of competitive displacement between Psyttalia humilis and D. tryoni, with both operating effectively in preferred habitats/seasons.
This species does not develop on Oriental fruit fly unless the latter has previously been attacked by another parasitoid.
Originally introduced to Hawaii by Silvestri in 1913 to control Ceratitis capitata, the medfly (Back and Pemberton 1915, Pemberton and Willard 1918, Back and Pemberton 1918, Willard and Mason 1937), this species continues to play an important role in suppression of populations of this pest in Hawaii (Wong et al. 1984, Wong and Ramadan 1987).
Methods for mass rearing this species for augmentative biological control have been published (Ramadan et al. 1989, Wong and Ramadan 1992), and production figures of over a million wasps per week have been achieved. Associated demographic work was published by Carey et al. (1988).
A listing of D. tryoni introductions in the New World for biological control is provided below summarized from Ovruski et al. (2000):
Argentina—-against Ceratitis capitata (Ovruski et al. 2003).
Puerto Rico—introduced in 1935-37 against Anastrepha suspensa, A. obliqua (Bartlett 1941).
Costa Rica—introduced in 1955 against Ceratitis capitata (Clausen 1978).
Mexico—introduced in 1988, 1994 against A. ludens.
Guatemala—introduced in 1995 against C. capitata (Sivinski et al. 2000).